The procedure was performed in a 15-year-old boy with Kearns-Sayre Syndrome. It is a mitochondrial myopathy, a rare genetic disease associated with abnormal functioning of mitochondria in the body's cells. It is characterized by drooping eyelids, retinal pigmentary degeneration, and symptoms such as nystagmus and double vision. And also sensorineural deafness, cardiac disorders - cardiomyopathy, conduction disorders including progressive atrioventricular heart block. There are also neurological disorders (e.g., weakening of the cerebellar structure), gastrointestinal disorders, endocrine disorders, and kidney failure. The disease progresses slowly, over decades. More symptoms appear and those already present slowly worsen.
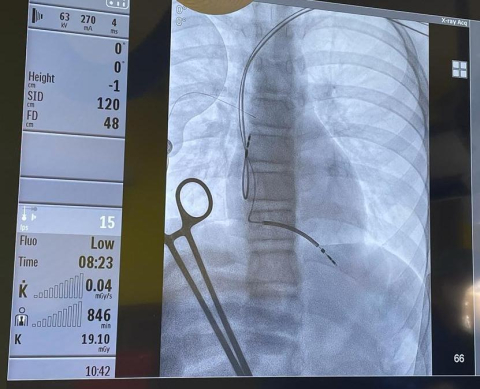
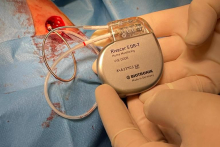
The boy’s Holter ECG monitoring recorded ventricular tachycardias and ECG showed sinus rhythm with previously undescribed bipolar block. Due to the high risk of malignant arrhythmias and conduction disturbances described in this disease, the decision was made to implant endocavitary leads, providing the patient with optimal safety both in case of ventricular fibrillation and possible complete block in this myopathy. The Rivacor 5 DR-T implanted in the Department of Pediatric Cardiology and General Pediatrics at UCC MUW is one of the most modern devices used in electrotherapy.
- The use of the latest technological solutions has made it possible to achieve a compromise between the size of the device and its service life. The Rivacor 5 DR-T is very thin, has a physiological shape to facilitate implantation and minimize tissue tension, and the battery life is one of the longest on the market. The use of as many as 4 discriminators and the maximum number of possible high-energy therapies, affects the safety and increases the probability of correct interpretation of arrhythmia - says Piotr Wieniawski, MD, PhD, one of the doctors performing the procedure.
Rivacor 5 DR-T is certified to safely perform 1.5T and 3T MRI. Additionally, it is equipped with BIOTRONIK's unique auto MRI algorithm that enables automatic reprogramming into MRI mode prior to the examination. This feature allows the patient to stay in MRI Setup Mode for a maximum of a short period of time, as the device will return to its original settings on its own when the examination is complete.
After the surgery, the boy received telemetry care in the Home Monitoring system allowing remote monitoring of heart function. Telemonitoring monitors the operation of the device daily and automatically notifies the medical team if there are any abnormalities. Professor Bożena Werner, Head of the Department of Pediatric Cardiology at the Medical University of Warsaw, emphasizes: This procedure is another step in the cooperation between our clinic and the 1st Department of Cardiology UCC MUW, headed by Prof. Marcin Grabowski. We currently perform a full spectrum of cardiac electrotherapy device implantations, including all types of devices that deliver high energy therapy.
The procedure was performed by a team composed of: Marcin Michalak, MD, PhD, Piotr Wieniawski, MD, PhD, Prof. Marcin Grabowski - proctor, anesthesiologist Izabela Pągowska, MD, PhD, specialist in anaesthetic nursing Mariusz Kiliszek, operating nurses Zofia Ryszkowska-Wójcik and Zofia Gulbicka. Technical support Ewelina Boczar and a team of cardiovascular research center technicians - Adam Bremer and Marta Burczaniuk. Dr Tomasz Książczyk and Dr Monika Jarecka participated in the diagnostic process of the patient.